
 |
 |
|
|
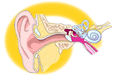
BAHA® (Bone Anchored Hearing Device) SystemWho can benefit from the BAHA® Examples of conditions helped by the BAHA® The BAHA® system is not a hearing aid. Instead, the system combines a sound processor (worn externally) with a small implanted titanium fixture implanted behind the ear. The system allows sound to be conducted through the bone of the skull directly to the inner ear (cochlea) rather than via the middle ear -- a process known as direct bone conduction. No component of the BAHA® system fits inside the ear or ear canal. The BAHA® does not rely on amplification to improve sound tone or quality. The BAHA® system is approved by the Food and Drug Administration (FDA) as a class II device.
Who can benefit from the BAHA® The BAHA® system is approved for use in children aged 5 or older for the the treatment of conductive or mixed hearing loss, as well as for unilateral sensorineural hearing loss, also known as Single Sided Deafness (SSD). The BAHA® system is placed in the mastoid bone behind the ear. As such, the system is a viable treatment for conductive or mixed hearing loss, as there is no in-the-ear component to aggravate further inner ear infections or block sound. Prior to the BAHA®, patients with SSD had only one available treatment -- the CROS (contralateral routing of signal) hearing aid, a treatment with limited performance due to its placement inside the only functioning ear. For those with SSD, the BAHA® system gives patients the opportunity to hear from both sides. For those under age five, the BAHA® Softband is available, using an adjustable elastic band to hold the sound processor comfortably against the skin. Examples of specific conditions helped by the BAHA® system
Currently, there are more than 20,000 BAHA® users worldwide. For more information regarding the BAHA® system, please visit www.entific.com. |
|||||||||||||||||||||||||||||
© 2006 Ohio Ear Institute, LLC |
||||||||||||||||||||||||||||||